
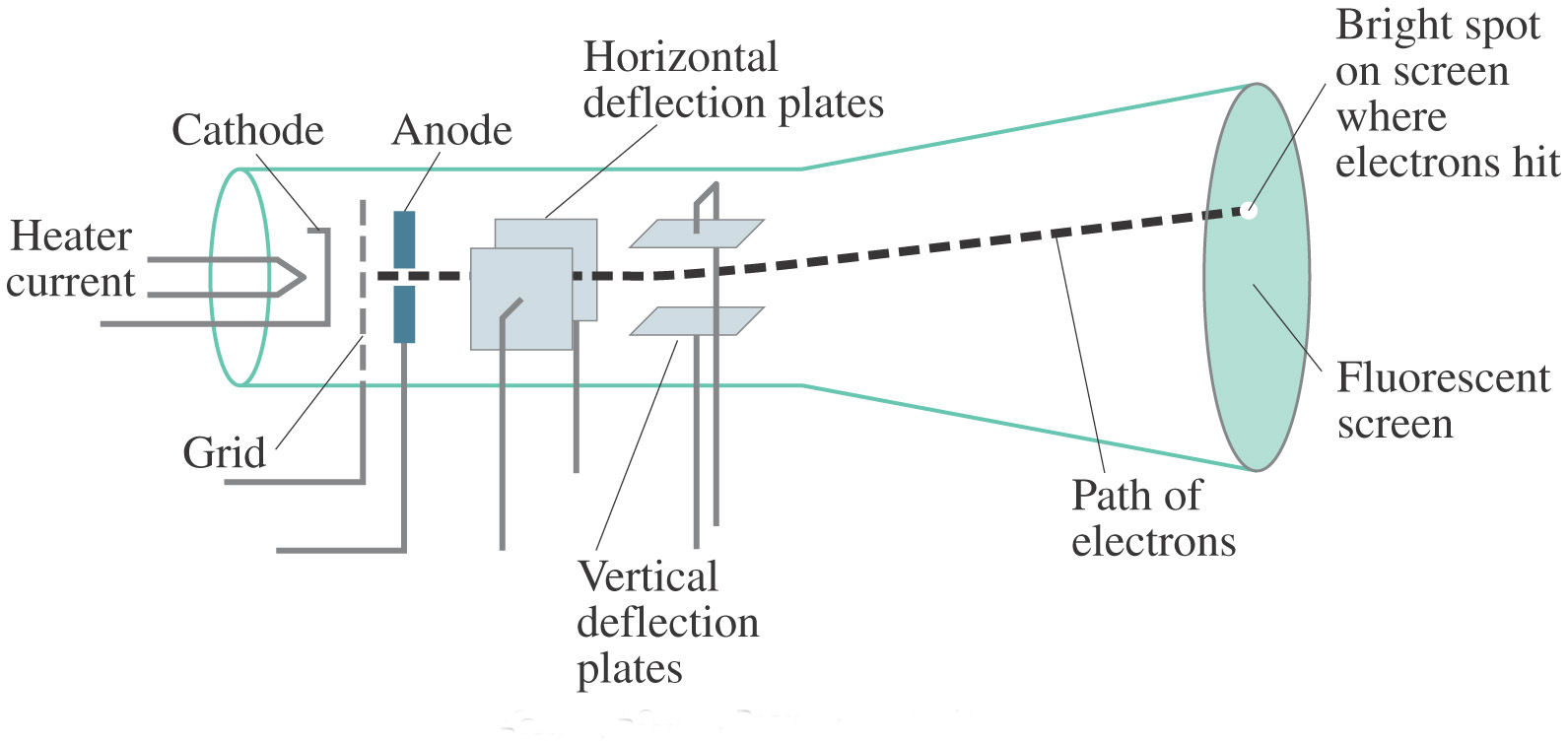
Our measurements yield a maximum impulsive force due to the electrons = (1.1 ± 0.3) × 10N], which is within a factor of two of Thomson's estimate, and which is more than two orders of magnitude smaller than the force that is responsible for the observed acceleration of the paddle wheel = (6 ± 2) × 10N]. We could then compare the force, which really acts on the wheel to produce the observed motion to the maximum impulsive force that is supplied by the electrons. We then measured the actual acceleration of the wheel in the CRT by video analysis of its motion and determined the moment of inertia of the wheel along with its mass and dimensions. The misconception was not laid to rest, however, and despite an effort in 1961 to draw attention to Thomson's original work and so remove the error from textbooks, the notion that a Crookes paddle wheel CRT demonstrates that electrons carry momentum continues to be taught in high school physics courses and wheel. In 1903 Thomson discounted Crookes' interpretation by calculating that the rate of momentum transfer (which he estimated at no more than 2×10 dyn, equivalent to 2×10 N) would be far too small to account for the observed motion of the wheel, instead attributing the motion to the radiometric effect. Crookes attributed the motion of the wheel to momentum transfer from the cathode rays (electrons) to the wheel, and interpreted the experiment as providing evidence that cathode rays were particles. 1) when connected to a high-voltage induction coil. Farnsworth invented a magnet which concentrated on the streamlet of electrons for fabricating a picture on the screen.In 1879, in the midst of the debate between English and continental scientists about the nature of cathode rays, William Crookes conducted an experiment in which a small mill or "paddle wheel" was pushed along tracks inside a cathode ray tube (CRT) (similar to that shown in Fig. But on that time, Campbell-Swinton vision did not come true. A.A Campbell got motivated by Braun’s oscilloscope, gave counselling that cathode ray tube can be used for forecast of video picture on the screen. In twentieth century, in the first two three decades researchers kept continuing their search for applications of the cathode ray tube technology. For scanning, the dot was passed across the screen which was visually represented by the electrical pulse generator. The typical cold cathode gas discharge X-ray tube found in schools is the. The cathode ray tube had applications in production of luminescence on a screen which was chemically affected on which the cathode rays were allowed to pass through the narrow aperture by focusing on the dot look alike beam. experiments in school science programs involve use of potential sources of. The introduction of Karl Ferdinand Braun’s oscilloscope ended the race of the search of all the researchers in the year 1897. Many researchers were thrilled to get to know the secret behind the cathode rays, while many where actually in search of the applications of the cathode ray tube experiment. As a result, the components of the discharge tube are charged negatively.Īpplications of the cathode ray tube experimentĭuring the time unknown, the cathode ray tubes has applications in the electron beam in which there was no consideration of inactivity but have high level of frequencies and for a small fraction of time it can be made visible.

By noting the places carefully where the fluorescence is observed, it was pointed out that the positive side was the sight where the deflections where noted.
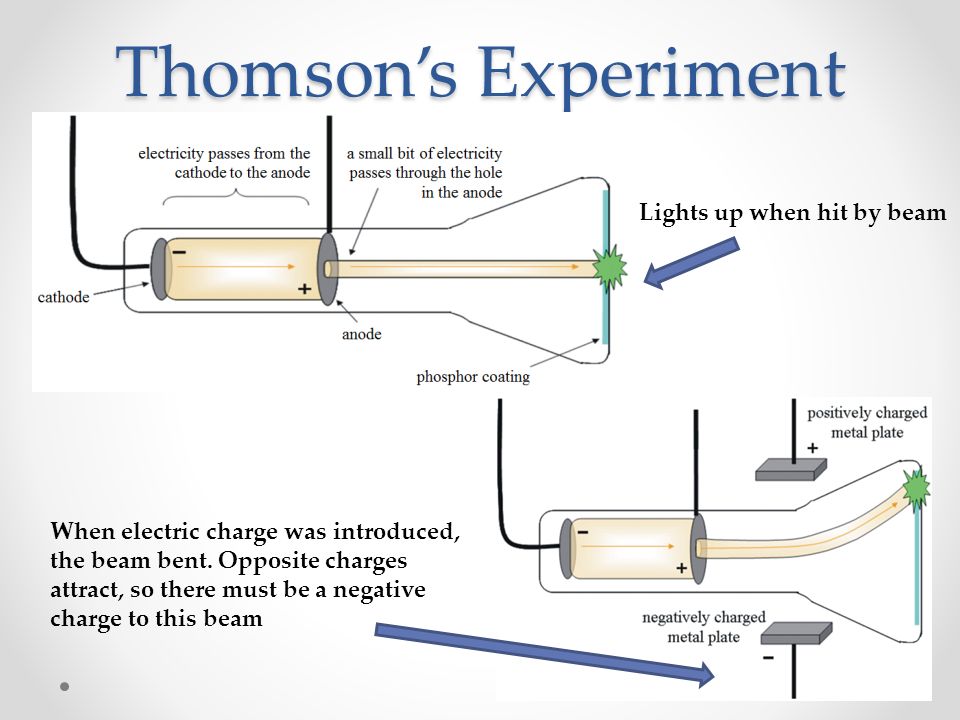
The metal pieces are supplied with a high voltage for air ionization to make it a good conductor of electricity.The set is made is such a way to give source of high voltage and draining the air to maintain low pressure inside of the tube.
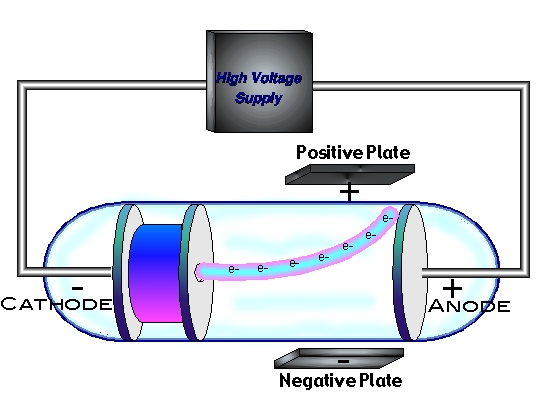
The air is completely drain out of the glass tube to keep the pressure of gas low. There is external voltage in connection with the two metal pieces. The set- up of the apparatus is done is manner such that there is in co-operation of a glass tube comprising of two metal pieces towards the opposite end which behaves as electrodes.


 0 kommentar(er)
0 kommentar(er)
